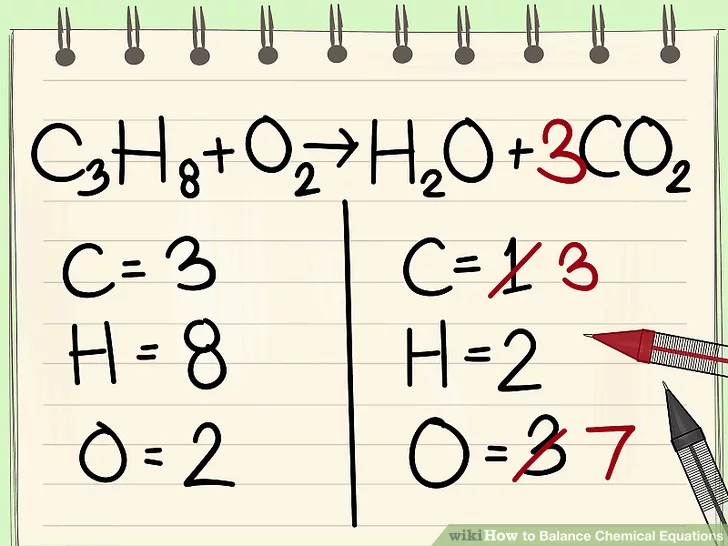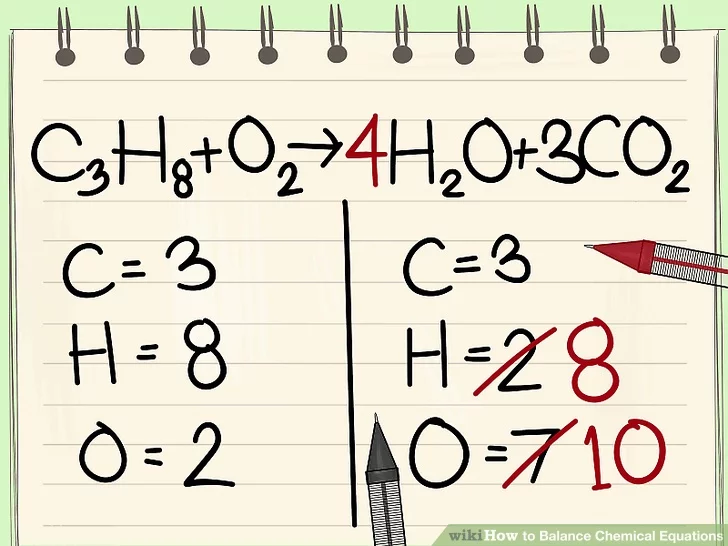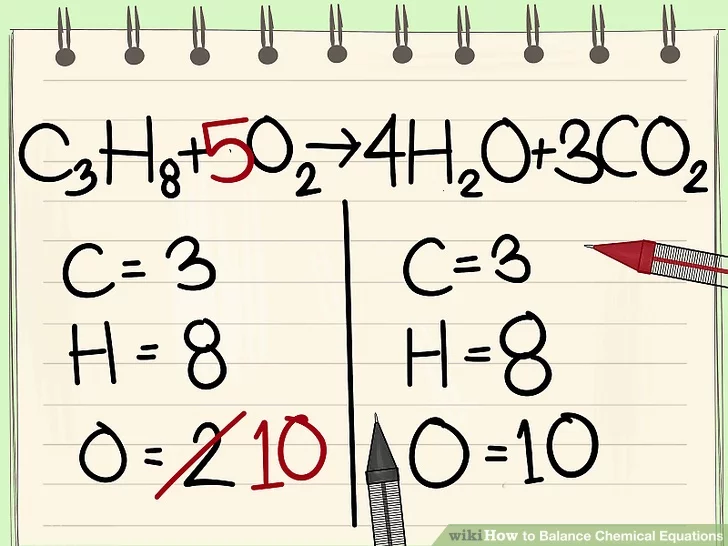THE BASIC FEASTURES
CHEMICAL REACTIONS OCCUR WHEN 2+ MOLECULES INTERACT WIF EACH OTHER ANND UNDERGO CERTAIN CHANGES
AH CHEMICAL REACTION REARRANGES THE ATOMS OF THE REACTANT TO CREATE A DIFFERENT SUBSTANCE AS A PRODUCT
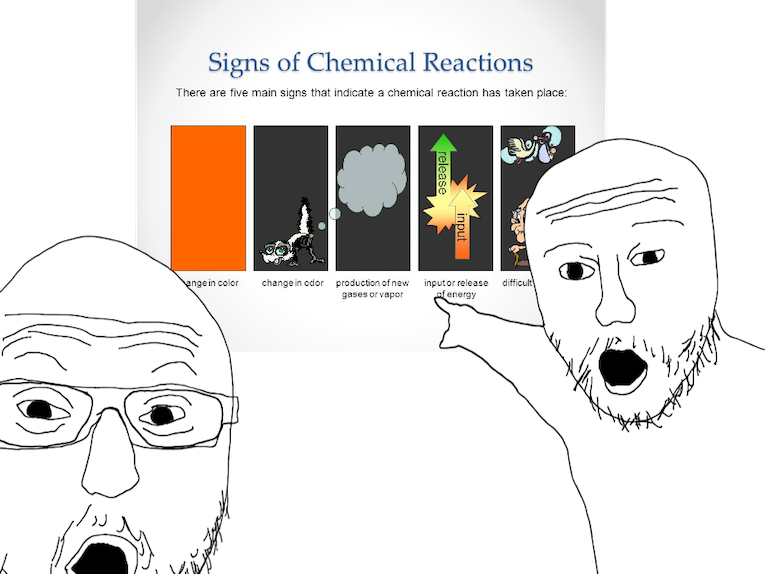
THESER R COMMON CHARACTERISITICS OF A CHEMICAL REACTIOON:
- Evolution Of A Gas
- Formation Of A Precipitate
- Change In Color
-
- Change In Temperature
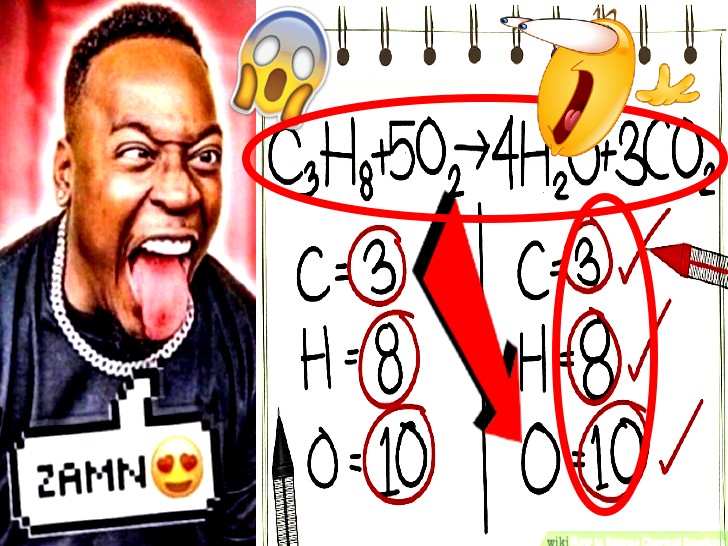
BALANCING
AN EQUATION IS BALANCED WHEN THE SAME NUMBER OF EACH ELEMENT IS REPRESENTED ON THE REACTANT AND THE PRODUCT SIDES
EQUATIONS MUST BE BALANCED TO ACCURATELY REFLECT THE LAW OF CONSERVATION OF MATTER
HERE IS AN EQUATION THAT IS NOT BALANCED
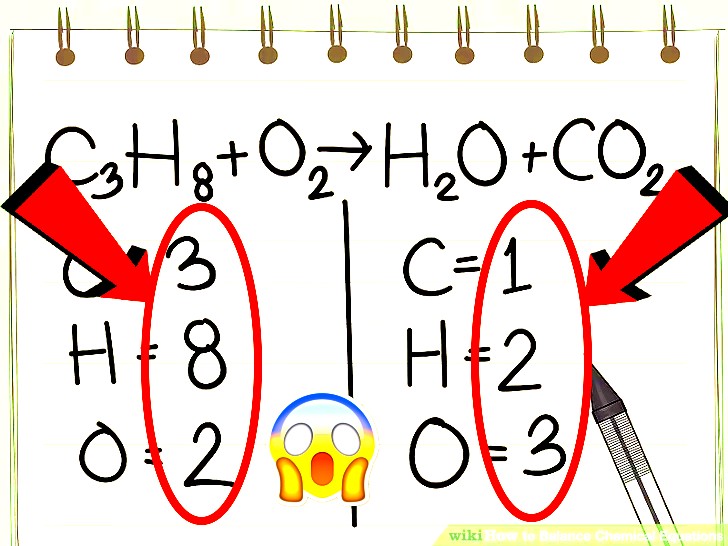
U SHOULD FIRST WRITE DOWN HOW MANY ATOMS THERE ARE FOR EACH ELEMENT. HERE THEY HAVE ALREADY DONE THAT
I JUST GO FROM LEFT TO RIGHT IDK SO LETS DO CARBON FIRST

OH NOOOOO THERES TOO MANY ON THE LEFT SIDE. LETS TIMES THE RIGHT ONE SO THE NUMBER OF CARBON ATOMS ARE EQUAL
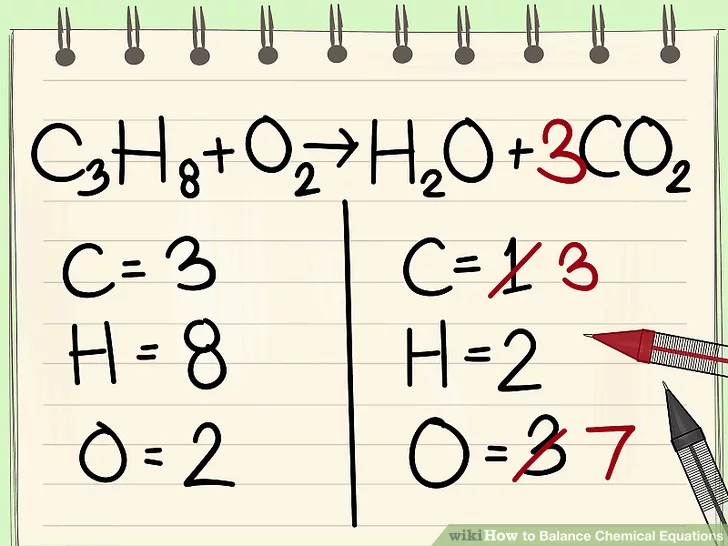
NOOOOO NOW THERES TOO MANY OXYGEN ATOMS (WIKIHOW DIDNT BALANCE OXYGEN IMMEDIATELY ANDI STOLE THESE PICS FROM THEM SO I GUESS WE WILL LITERALLY JUST LEAVE IT THERE)
DO THE THING WE DID WITH CARBON WITH HYDROGEN
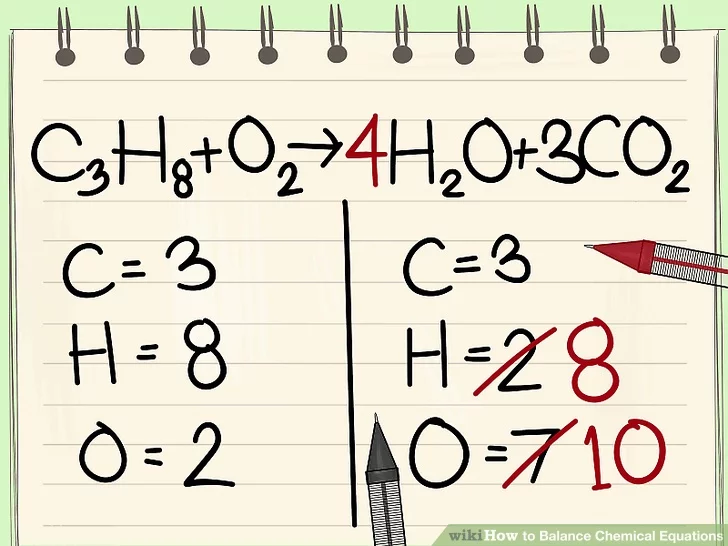
NOW WE CAN FINALLY FIX OXYGEN
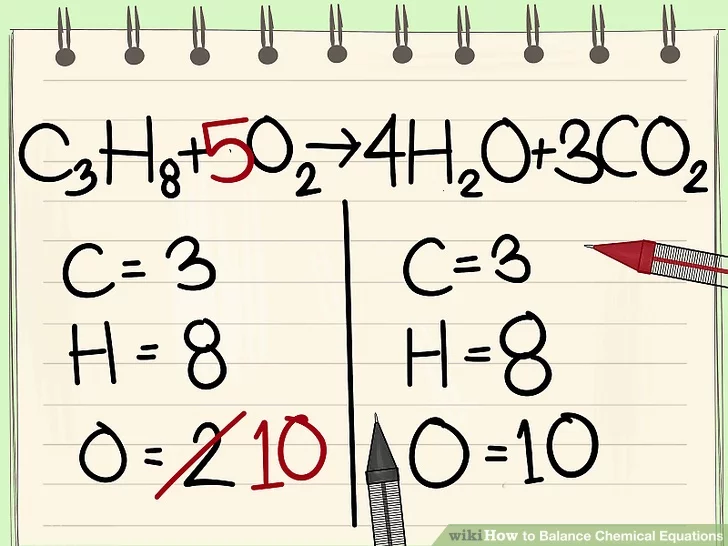
THE CHEMICAL EQUATION IS BALANCED!!!!